Sample Projects
Go back to:
Or learn more on the general development and behaviour of frog tadpoles.
Here are some projects we have completed recently. More projects can be found at my colleagues’ website (tadpole research at Bristol).
Mechanisms underlying the recruitment of inhibitory interneurons in fictive swimming in developing Xenopus laevis tadpoles (Ferrario et al 2023)
Developing spinal circuits generate patterned motor outputs while many neurons with high membrane resistances are still maturing. In the spinal cord of hatchling frog tadpoles of unknown sex, we found that the firing reliability in swimming of inhibitory interneurons with commissural (cINs) and ipsilateral ascending axons (aINs) was negatively correlated with their cellular membrane resistance. The predictability of the recruitment order of these developing interneurons in swimming by cellular input resistances is opposite to that in the motor-strength based recruitment depicted by Henneman’s size principle.
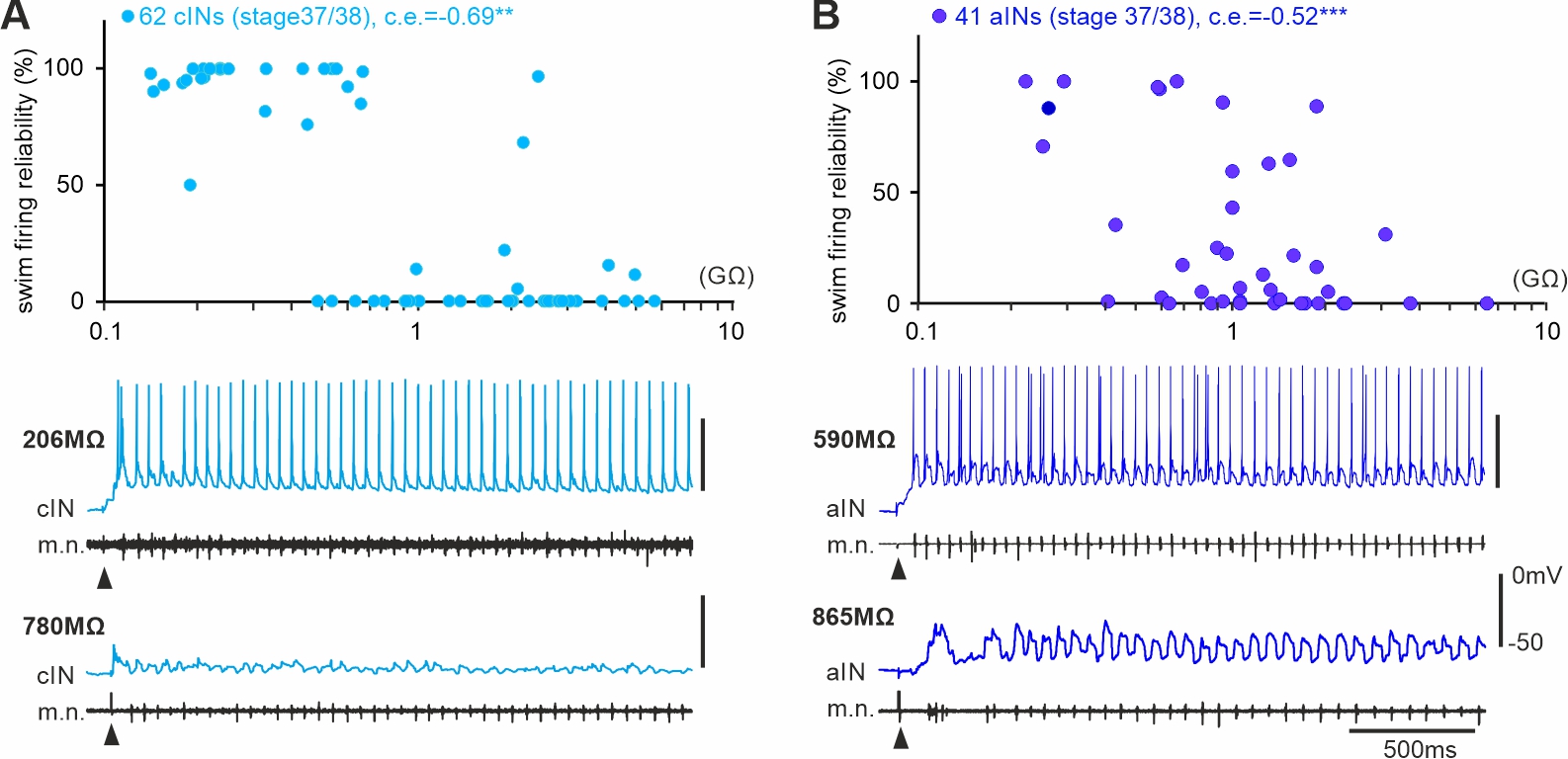
Self-absorbed comments: This form of recruitment/integration order in early development is progressive potentially with neuronal acquisition of mature electrical and synaptic properties. When motor control becomes more fine-tuned in later development, this may be replaced by the motor-strength based recruitment.
The early development and physiology of Xenopus laevis tadpole lateral line system (Saccomanno et al., 2021)
Aquatic vertebrates like fish and amphibians use the distributed mechanosensory lateral line system to sense hydrodynamic disturbances, which could help them to avoid predation, detect prey or in rheotaxis. Although the lateral line system of tadpoles at two days old is still developing, the tadpole can respond to suction close to its head with reliable turning followed by swimming. We have characterised the afferent and efferent activities of the lateral system.
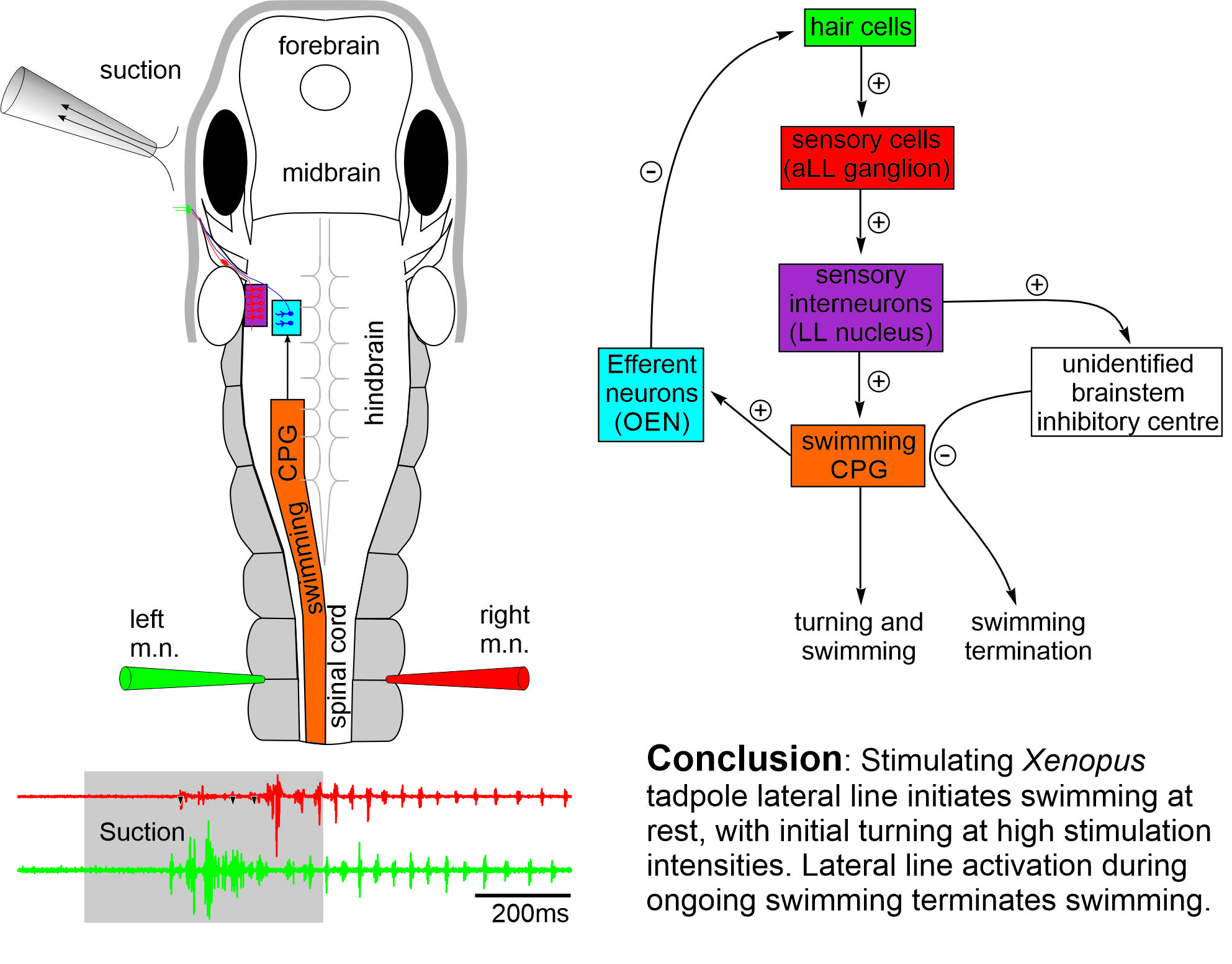
Self-absorbed comments: Escape behaviour in fishes are well known to be mediated by activating the large Mauthner cells in the hindbrain with short latencies in tens of ms. The tadpole turning behaviour in contrast has a latency of around 140 ms, suggesting a different escape circuit structure.
Single hindbrain cells start tadpole swimming (Li & Soffe 2019. Front Cell Neurosci)
Unlike in invertebrates, it is rare to be able to change animal behaviour by activating single neurons in the vertebrate central nervous system. Among a large sample of neurons in frog tadpole hindbrain, we have recorded several excitatory neurons a single spike of which could start tadpole swimming repeatedly. Further analyses suggest that this initial excitation is amplified by other neurons of the same type before the whole swimming network becomes active. These powerful individual neurons may express the molecular marker of CHX10.
Self-absorbed comments: The presence of powerful individual neurons can ease our pain in understanding their functions in behaviour, since it is normally difficult to earmark and control the activity of neuronal populations of the same type. However, their existence also suggests big redundancies in the neuronal network, the evolutional advantages of which remain to be explored.
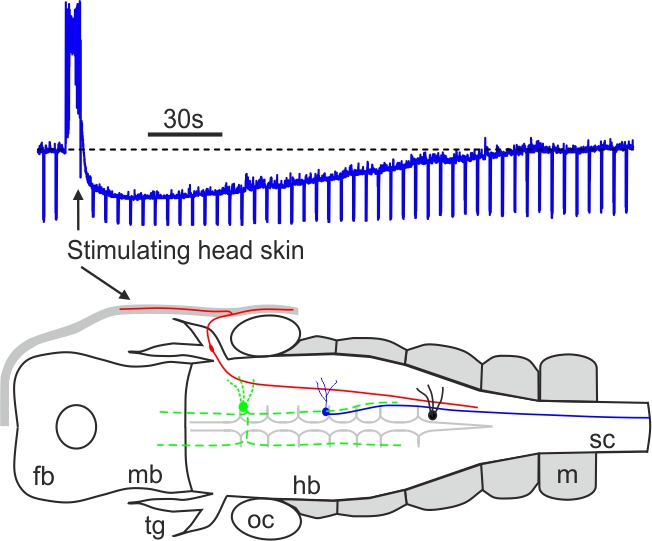
Mechanosensory stimulation evokes acute concussion-like behaviour (Li 2017, eNeuro)
Most vertebrates have concussion responses when their heads are hit suddenly by heavy objects, rendering the animals momentarily motionless and often unconscious. In Xenopus Laevis tadpoles, we find that concussion-like behaviour can be induced reliably by mechanosensory stimulation to the head skin. The head skin stimulation then activates some cholinergic neurons in the brainstem to inhibit the tadpole motor circuit. The results provide a potential explanation why concussion in vertebrates often recovers spontaneously without sustaining clear physical injury to the brain and some acute symptoms of concussion can be a neurophysiological response to specific sensory stimulation.
Self-absorbed comments: Apparently animal forward movement needs to be stopped when its head bumps into solid objects. It remains an open question if concussion is an over-blown stopping response or the loss of motor control is just a consequence of concussion.
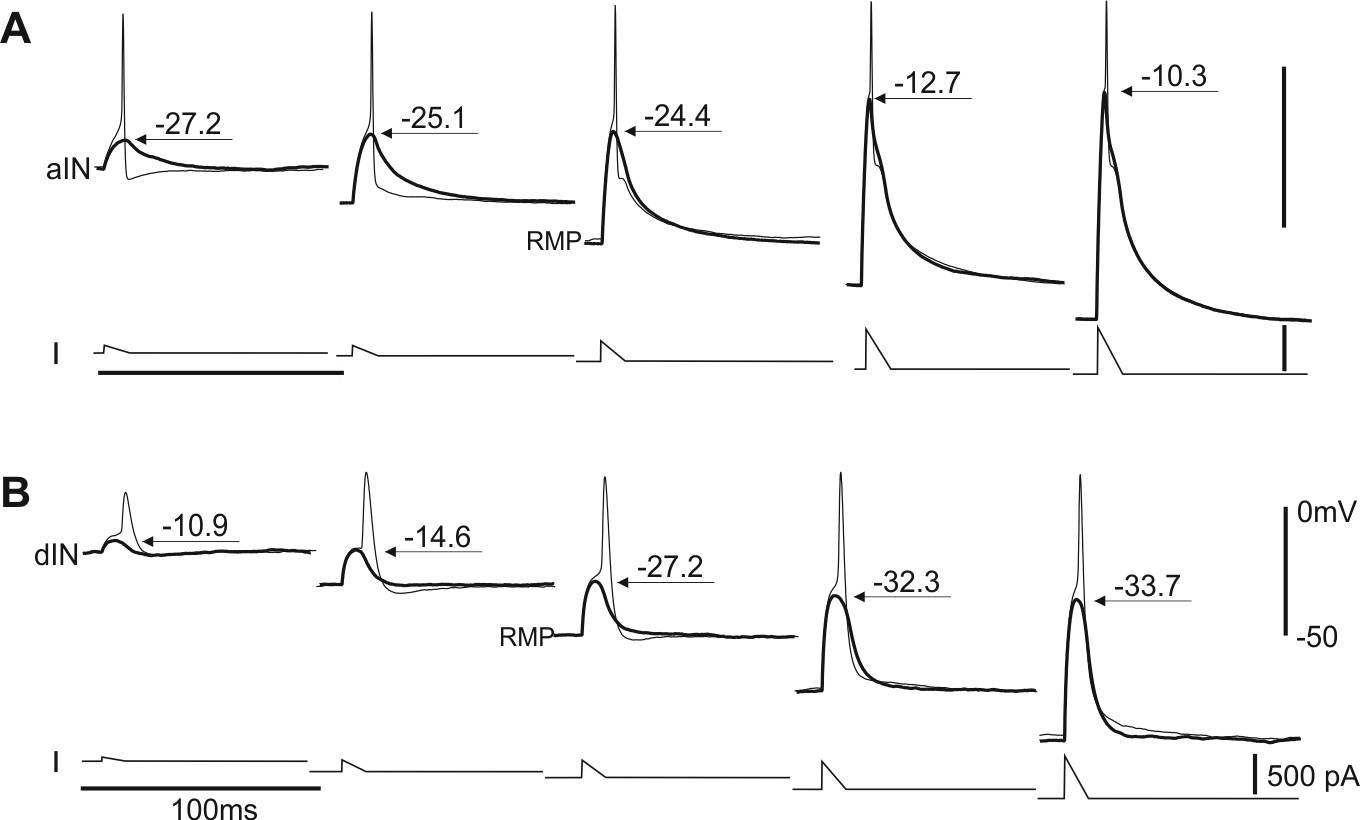
Intrinsic properties enable motor program switches (Li 2015, J Neurosci)
Many neural circuits are capable of producing multiple, patterned outputs. This is because the synaptic connections among some network neurons are “ubiquitous” but susceptible to fast reconfiguration when sensory inputs or neuromodulatory states change. In this study, I have identified that certain ion channels in the tadpole motor circuit can quickly shift neuronal firing thresholds depending on network excitation levels. Neuron-type specific expression of the channels then allows selective recruitment/depression of different neurons, dynamic configuration of the circuit and potential switching between motor programmes (swimming and struggling in tadpoles).
Self-absorbed comments: In line with many invertebrate studies, this work shows that functional circuits can be very different from the synaptic connection maps. Also, it implies “ubiquitous” synaptic connections may be necessary for multiple functional networks.
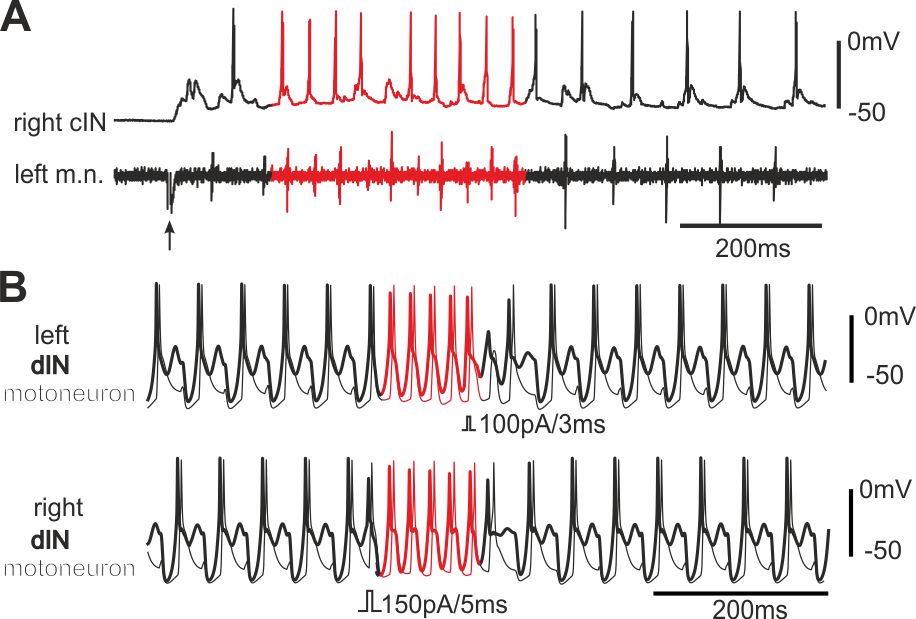
The same neural circuit generates two rhythms (Li et al 2014, J Neurosci)
Antiphase oscillations and synchrony are arguably the two most common neural rhythms in the CNS. We have found that both rhythms are intrinsically embedded in the same tadpole swimming circuit, which comprises of two half-centres coupled by reciprocal inhibition. The antiphase swimming rhythm is the stable output in normal conditions. Modelling predicts that synchrony can be converted to a stable regime by delaying the arrival of inhibition between the half-centres.
Self-absorbed comments: This research shows that the same central pattern generator based on post-inhibitory rebound can generate two different rhythms without circuit reconfiguration.
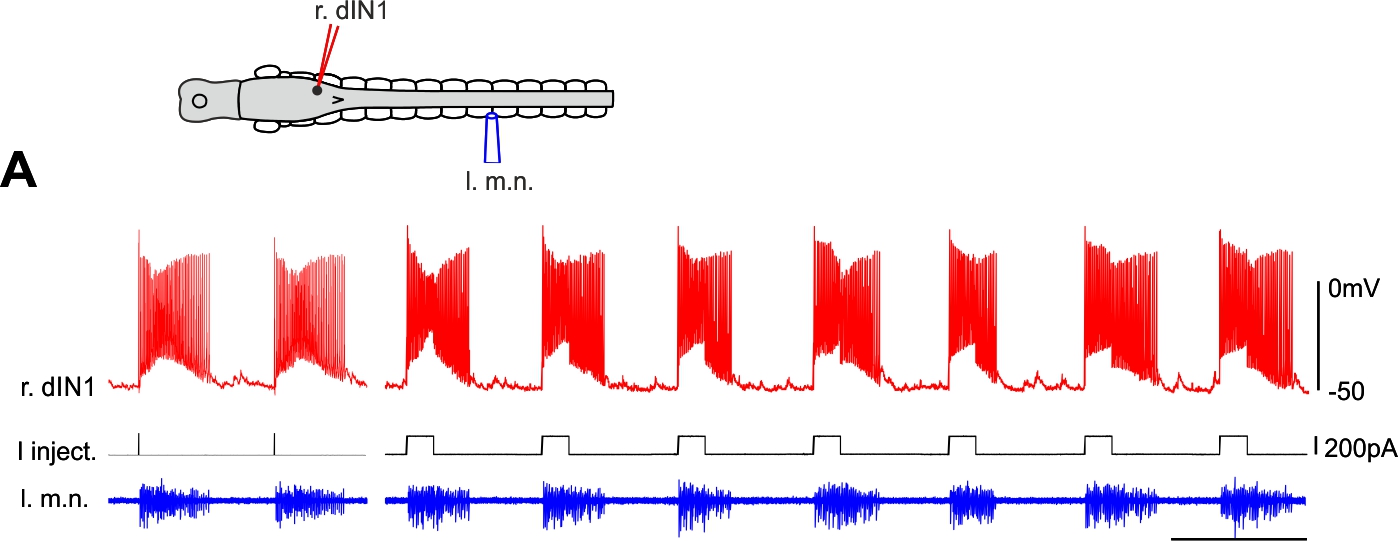
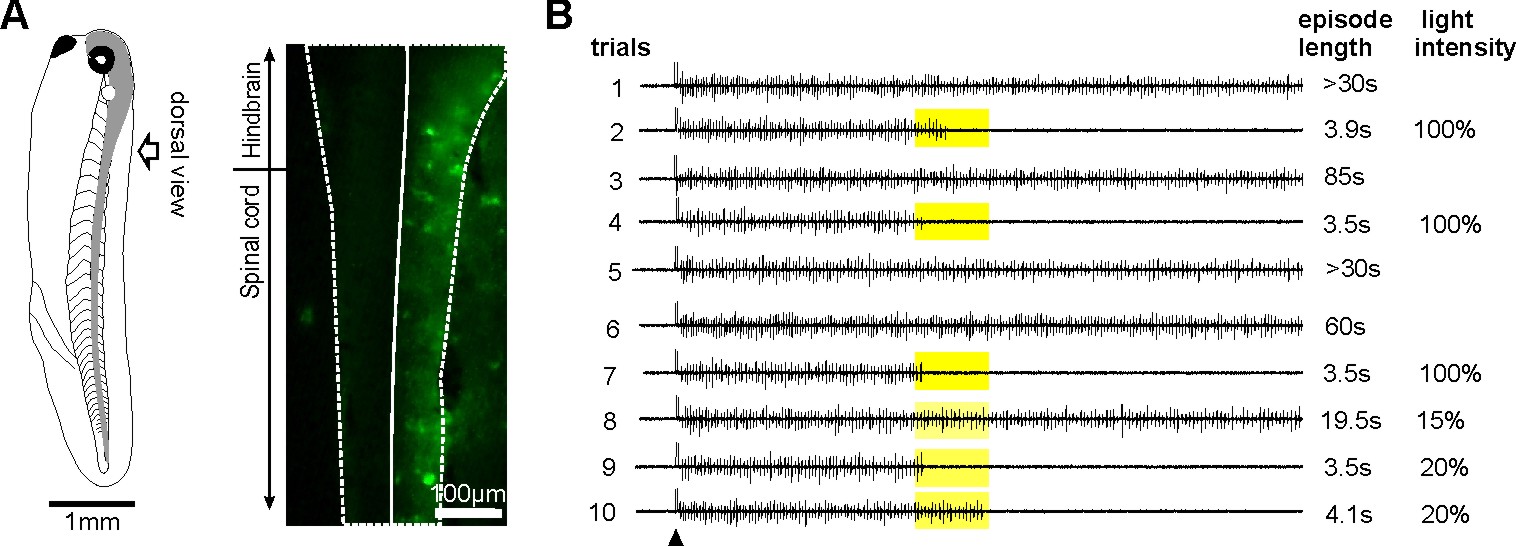
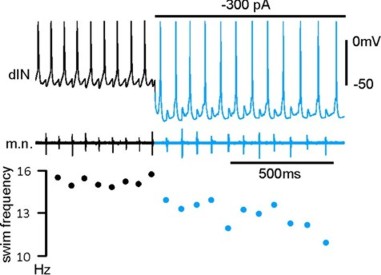
Normal tadpole swimming operates on rebound from reciprocal inhibition (Moult et al., 2013, Neuron)
We wanted to identify which mechanism, rebound or pace-maker firing, dominates normal swimming activity. Using two fast silencing methods (optogenetics and large –DC injections into dINs, see other projects), we found that tadpole spinal activity on one side is dependent on the other side being active. Analyses show:
- the failure of activity result from depression of reciprocal inhibition
- pace-maker driven swimming-like rhythms requires a recovery period
Self-absorbed comments: This work lends strong support to the critical role for reciprocal inhibition in locomotor control proposed by Graham Brown (Brown’s hypothesis, Brown 1911, 1914).
The control of tadpole swimming frequency by excitation and inhibition (Li & Moult 2012, J Neurosci)
Tadpole swimming rhythms are maintained either from rebound or pace-maker firing in the excitatory interneurons, dINs. By analysing how excitation and inhibition affect dIN rebound firing speed, we have identified that:
- Background excitation directly determines swimming frequency
- Strong phasic inhibition in certain cases can speed up swimming
Self-absorbed comments: It is rare in vertebrate central nervous system that changing the activity of single neurons can affect behavior. Fortuitously, single dIN firing can affect swimming. This makes testing many aspects of tadpole swimming control straightforward.
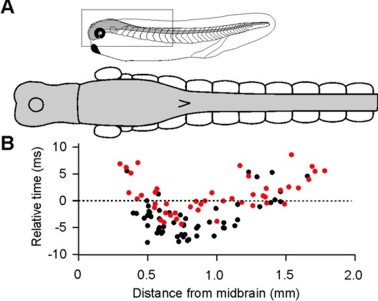
Identification of neurons that fire first in a behavior (Soffe et al., 2009, J Physiol)
The neurons that fire first during certain behaviour can imply their critical, driving roles. Using loose-patch recordings, we recorded many neurons from single tadpoles. Analyses show that the earliest-firing neurons during a swimming cycle are the excitatory interneurons (dINs), located in the caudal hindbrain and rostral spinal cord region. While dIN EPSPs could directly drive the activity of other neurons, dIN firing itself appeared to rely on reciprocal inhibition (rebound).
Self-absorbed comments: It is uncommon to be able to find the neurons that fire first in a function mediated by the central nervous system. Though the approach we have used here is not ideal, the results are clear. The properties of dINs thus potentially hold many keys to swimming control.